Sodium hydroxide is of great importance for industry, which is why it is widely used. Caustic, or caustic soda, is used in almost all areas of human life - from chemical production to the food industry. Despite its corrosive properties, this alkali is registered as a food additive E524. This does not mean that it is not harmful to health at all, although caustic soda is not dangerous in minimal doses.
Sodium hydroxide is extremely dangerous in excess dosages
History of the discovery of the substance
The first mention of a compound whose properties are reminiscent of caustic soda appears in ancient times. Even the Bible contains some information about the substance neter, extracted from the Egyptian lakes. Presumably this was caustic soda.
Aristotle, Plato and other ancient Greek and Roman philosophers and scientists also mentioned the substance nitrum, which was extracted from natural reservoirs and sold in the form of large differently colored pieces (black, gray, white). After all, nothing was known about purification methods at that time, so it was not possible to separate the compound from the coal that polluted it.
Soap making was introduced in 385 BC. The process was based on caustic soda. Its formula, of course, was not yet known, but this did not stop it from being extracted from the ash of plants of the Solyanka genus, from lakes and used for cleaning household items, washing clothes, and making various soaps.
A little later, the Arabs learned to add essential oils and aromatic substances to the product. Then the soap became beautiful and smelling good. The active development of soap-making processes and technologies began.
Until the 17th century, caustic soda, the properties of which were widely used, remained unstudied as a chemical compound. It was combined with substances such as soda, potassium hydroxide, potassium and sodium carbonates. All of them were called caustic alkalis.
Later, the scientist Duhamel du Monceau was able to prove the difference between these substances and divided them into alkalis and salts. Since then, caustic soda received its true and constant name to this day.
How can you use caustic soda at home?
At home, caustic soda is usually used to clean dishes (pots, pans and kettles) from carbon deposits, old grease stains and scale.
It is also used to clean sewer drains. It corrodes everything organic, so it easily removes all fat, protein and other deposits from the surface of the pipes.
So, in order to get rid of blockages in pipes, you need to shake the container in which caustic soda is stored thoroughly. Then you need to pour or pour (depending on what form the caustic is in: liquid or granular) 2-3 tablespoons of the substance directly into the drain, and pour 1 glass of hot water on top. You need to wait two hours for the reaction to occur, and then you need to rinse the drain with a strong stream of water.
Caustic soda is also used to clean pipelines. It is added to the water when testing pipes using a shock wave. This solution helps clean them from rust and scale that has formed on the inner surface of the pipes.
Synonyms of names
It should be noted that the name of this substance is not the same and has several synonyms. In total there are 6 different options:
- sodium hydroxide;
- caustic soda;
- caustic soda;
- sodium lye;
- caustic;
- caustic alkali.
This compound is called caustic soda in common people and industry. In chemical syntheses, it is more correct to say sodium alkali or caustic soda. This does not change the formula. The most common name is caustic. The correct name from the point of view of the systematic nomenclature of substances is sodium hydroxide.
Cesspools
Caustic soda is classified as a gentle substance for cleaning cesspools, but its use is possible only if all conditions are met:
- Septic tanks must be sealed, since the substance, when reacting, can harm the soil and the environment.
- To avoid an explosive situation, the ventilation system must operate without interruption.
- You cannot start cleaning without a protective suit, gloves, and goggles.
Sewage pits are cleaned using a caustic alkali solution.
The portion of soda indicated in the instructions is diluted in cold water, stirring constantly. At the peak of its activity, the composition lasts for about four minutes, so the product is immediately used for its intended purpose.
Chemical formula and molecule structure
If we consider this substance from a chemical point of view, it will consist of two ions: sodium cation (Na+) and hydroxide anion (OH-). Bonding with each other due to the electrostatic attraction of differently charged particles, these ions form caustic soda. The empirical formula will be NaOH.
The hydroxo group is formed by a covalent polar bond between oxygen and hydrogen, while it is held by an ionic bond with sodium. In solution, alkali completely dissociates into ions, being a strong electrolyte.
Physical characteristics of caustic soda
Molecular weight: 39.997 g/mol
Boiling point of a 44% solution is 140-142°C
The freezing point of liquid caustic soda, grade RD, grade 1, with a concentration of 44% is +7°C. The dependence of the freezing point of caustic soda is shown in Fig. 1:
The density of the 44% solution is 1.468 g/cm3. The densities of aqueous solutions of caustic soda are presented in Fig. 2:
Caustic soda solution is a strong electrolyte. Electrical conductivity is shown in Fig. 3:
Laboratory method of obtaining
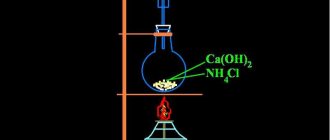
Industrial and laboratory methods for producing caustic soda closely overlap. It is often produced in small quantities by chemical and electrochemical methods in smaller installations than in industrial facilities. And tons of the substance are produced using the same methods in huge columns of electrolyzers.
There are several main methods for synthesizing caustic in the laboratory.
- Ferritic method. It consists of two main stages: in the first, sodium carbonate and iron (III) oxide are sintered under the influence of high temperature. As a result, sodium ferrite (NaFeO2) is formed. In the second stage, it is exposed to water and decomposes to form sodium hydroxide and a mixture of iron and water (Fe2O3*H2O). The resulting caustic soda is evaporated from the solution to white crystals or flakes. Its purity is approximately 92%.
- Lime method. It consists of a reaction between sodium carbonate and calcium hydroxide (slaked lime) to form calcium carbonate and caustic soda. The reaction is carried out at a temperature of 80°C. Since the resulting salt precipitates, it is easily separated. The remaining solution is evaporated and sodium alkali is obtained.
- Diaphragm and membrane production methods. Based on the operation of the electrolyzer installation. It is fed with a solution of table salt (NaCL), which undergoes electrolysis to form free chlorine gas and the desired caustic product. The difference between these methods is that with the diaphragm method, the main structural part of the device is an asbestos diaphragm (cathode). With the membrane method, the cathode and anode spaces are separated by a special membrane.
This is how sodium hydroxide is obtained in the laboratory, choosing the most financially advantageous option. It is also, as a rule, less energy-consuming.
Chemical properties of sodium hydroxide. Chemical reactions of sodium hydroxide:
Sodium hydroxide is a chemically active substance, a strong chemical base.
Aqueous solutions of NaOH have a strong alkaline reaction (pH of a 1% solution = 13.4).
The chemical properties of sodium hydroxide are similar to those of other alkali metal hydroxides. Therefore, it is characterized by the following chemical reactions:
1. reaction of sodium hydroxide with sulfur:
3S + 6NaOH → 2Na2S + Na2SO3 + 3H2O (t = 50-60 °C).
The reaction produces sodium sulfide, sodium sulfite and water. In this case, sodium hydroxide is used as a starting material in the form of a diluted solution.
2. reaction of sodium hydroxide with chlorine:
2NaOH + Cl2 → NaCl + NaClO + H2O.
The reaction produces sodium chloride, sodium hypochlorite and water. In this case, sodium hydroxide is used as a starting material in the form of a cold diluted solution.
The reactions of sodium hydroxide with other halogens proceed similarly.
3. reaction of sodium hydroxide with aluminum:
2Al + 6NaOH → 2NaAlO2 + 3H2 + 2Na2O (t = 450 °C).
The reaction produces sodium aluminate, hydrogen and sodium oxide.
4. reaction of sodium hydroxide with aluminum and water:
2Al + 2NaOH + 6H2O → 2Na[Al(OH)4] + 3H2.
As a result of the reaction, sodium tetrahydroxoaluminate and hydrogen are formed. In this case, sodium hydroxide is used as a starting material in the form of a concentrated solution.
This reaction was used in the first half of the 20th century in aeronautics: to fill balloons and airships with hydrogen in the field, since this reaction does not require sources of electricity, and the starting reagents for it can be easily transported.
5. reaction of sodium hydroxide with zinc:
Zn + 2NaOH → Na2ZnO2 + H2 (t = 550 °C).
As a result of the reaction, sodium zincate and hydrogen are formed.
6. reaction of sodium hydroxide with zinc and water:
Zn + 2NaOH + 2H2O → Na2[Zn(OH)4] + H2.
As a result of the reaction, sodium tetrahydroxozincate and hydrogen are formed. In this case, sodium hydroxide is used as a starting material in the form of a concentrated solution.
7. reaction of sodium hydroxide with phosphoric acid:
H3PO4 + NaOH → NaH2PO4 + H2O.
As a result of the reaction, sodium dihydrogen orthophosphate and water are formed. In this case, the following starting materials are used: phosphoric acid in the form of a concentrated solution, sodium hydroxide in the form of a diluted solution.
8. reaction of sodium hydroxide with nitric acid:
NaOH + HNO3 → NaNO3 + H2O.
As a result of the reaction, sodium nitrate and water are formed. In this case, nitric acid is used as a starting material in the form of a diluted solution.
9. reaction of sodium hydroxide with nitric acid:
NaOH + HNO3 → NaNO3 + H2O.
As a result of the reaction, sodium nitrate and water are formed. In this case, nitric acid is used as a starting material in the form of a diluted solution.
The reactions of sodium hydroxide with other acids proceed similarly.
10. reaction of sodium hydroxide with hydrogen sulfide:
H2S + 2NaOH → Na2S + 2H2O,
H2S + NaOH → NaHS + H2O.
As a result of the reaction, in the first case, sodium sulfide and water are formed, in the second, sodium hydrosulfide and water. In this case, sodium hydroxide in the first case is used as a starting substance in the form of a concentrated solution, in the second case - in the form of a diluted solution.
11. reaction of sodium hydroxide with hydrogen fluoride:
HF + NaOH → NaF + H2O,
2HF + NaOH → NaHF2 + H2O.
As a result of the reaction, in the first case, sodium fluoride and water are formed, in the second, sodium hydrofluoride and water. In this case, sodium hydroxide and hydrogen fluoride in the first case are used as a starting material in the form of a diluted solution, in the second case, hydrogen fluoride is used in the form of a concentrated solution.
12. reaction of sodium hydroxide with hydrogen bromide:
HBr + NaOH → NaBr + H2O.
As a result of the reaction, sodium bromide and water are formed. In this case, sodium hydroxide and hydrogen bromide are used as a starting material in the form of a diluted solution.
13. reaction of sodium hydroxide with hydrogen iodide:
HI + NaOH → NaI + H2O.
As a result of the reaction, sodium iodide and water are formed. In this case, sodium hydroxide is used as a starting material in the form of a diluted solution.
14. reaction of sodium hydroxide with zinc oxide:
ZnO + 2NaOH → Na2ZnO2 + H2O (t = 500-600 °C).
Zinc oxide is an amphoteric oxide. As a result of the reaction, sodium zincate and water are formed.
15. reaction of sodium hydroxide with zinc oxide and water:
ZnO + NaOH + H2O → Na[Zn(OH)3] (t = 100 °C),
ZnO + 2NaOH + H2O → Na2[Zn(OH)4] (t = 90 °C).
Zinc oxide is an amphoteric oxide. As a result of the reaction, in the first case, sodium trihydroxozincate and water are formed, in the second case, sodium tetrahydroxozincate. In this case, sodium hydroxide is used as a starting substance in the first case in the form of a 40% diluted solution, in the second - in the form of a 60% diluted solution.
16. reaction of sodium hydroxide with aluminum oxide:
Al2O3 + 2NaOH → 2NaAlO2 + H2O (t = 900-1100 °C).
Aluminum oxide is an amphoteric oxide. As a result of the reaction, sodium aluminate and water are formed.
17. reaction of sodium hydroxide with aluminum oxide and water:
Al2O3 + 6NaOH + 3H2O → 2Na3[Al(OH)6],
Al2O3 + 2NaOH + 3H2O → 2Na[Al(OH)4].
Aluminum oxide is an amphoteric oxide. As a result of the reaction, in the first case, sodium hexahydroxoaluminate is formed, in the second case, sodium tetrahydroxoaluminate. In this case, sodium hydroxide is used as a starting material in the second case in the form of a concentrated hot solution.
18. reaction of sodium hydroxide with iron oxide:
Fe2O3 + 2NaOH → 2NaFeO2 + H2O (t = 600 °C, p).
Iron oxide is an amphoteric oxide. As a result of the reaction, sodium ferrite and water are formed. The reaction occurs when the starting substances fuse.
The reactions of sodium hydroxide with other amphoteric oxides proceed similarly.
19. reaction of sodium hydroxide with carbon monoxide (carbon dioxide):
NaOH + CO2 → NaHCO3.
As a result of the reaction, sodium bicarbonate is formed.
20. reaction of sodium hydroxide with sulfur oxide:
SO2 + NaOH → NaHSO3.
As a result of the reaction, sodium hydrosulfite is formed. In this case, sodium hydroxide is used as a starting material in the form of a diluted solution.
21. reaction of sodium hydroxide with silicon oxide:
2NaOH + SiO2 → Na2SiO3 + H2O (t = 900-1000 °C),
4NaOH + SiO2 → Na4SiO4 + 2H2O.
As a result of the reaction, in the first case, sodium silicate and water are formed, in the second case, sodium orthosilicate and water. In this case, sodium hydroxide is used as a starting substance in the second case in the form of a concentrated solution.
22. reaction of sodium hydroxide with aluminum hydroxide:
Al(OH)3 + NaOH → NaAlO2 + 2H2O (t = 1000 °C),
Al(OH)3 + NaOH → Na[Al(OH)4].
Aluminum hydroxide is an amphoteric base. As a result of the reaction, in the first case, sodium aluminate and water are formed, in the second case, sodium tetrahydroxyaluminate. In this case, sodium hydroxide is used as a starting substance in the second case in the form of a concentrated solution.
23. reaction of sodium hydroxide with zinc hydroxide:
Zn(OH)2 + 2NaOH → Na2[Zn(OH)4].
Zinc hydroxide is an amphoteric base. As a result of the reaction, sodium tetrahydroxozincate is formed. In this case, sodium hydroxide is used as a starting material in the form of a concentrated solution.
24. reaction of sodium hydroxide with iron hydroxide:
Fe(OH)3 + 3NaOH ⇄ Na3[Fe(OH)6].
Iron hydroxide is an amphoteric base. As a result of the reaction, sodium hexahydroxoferrate is formed.
The reactions of sodium hydroxide with other amphoteric hydroxides proceed similarly.
25. reaction of sodium hydroxide with ferrous sulfate:
FeSO4 + 2NaOH → Fe(OH)2 + Na2SO4 (kat = N2).
As a result of the reaction, iron hydroxide and sodium sulfate are formed.
26. reaction of sodium hydroxide with copper chloride:
CuCl2 + 2NaOH → Cu(OH)2 + 2NaCl.
As a result of the reaction, copper hydroxide and sodium chloride are formed. In this case, sodium hydroxide is used as a starting material in the form of a diluted solution.
27. reaction of sodium hydroxide with lead nitrate:
Pb(NO3)2 + 2NaOH → Pb(OH)2 + 2NaNO3.
As a result of the reaction, lead hydroxide and sodium nitrate are formed. In this case, sodium hydroxide is used as a starting material in the form of a diluted solution.
28. reaction of sodium hydroxide with aluminum chloride:
AlCl3 + 3NaOH → Al(OH)3 + 3NaCl.
As a result of the reaction, aluminum hydroxide and sodium chloride are formed. In this case, sodium hydroxide is used as a starting material in the form of a diluted solution.
The reactions of sodium hydroxide with other salts proceed similarly.
Synthesis in industry
How is such a substance as caustic soda produced in industry? Liquid and solid caustic soda is most often extracted using the electrochemical method. It is based on the electrolysis of a solution of the natural mineral halite, the vast majority of which is formed by table salt.
The main feature of this synthesis is that the by-products, along with caustic soda, are gaseous chlorine and hydrogen. The process is carried out in any of three options:
- diaphragm electrolysis on a solid cathode;
- with a liquid mercury cathode;
- membrane with a solid cathode.
The vast majority of caustic soda produced in the world is formed using the membrane method. The resulting alkali has a fairly high level of purity.
Areas of use
There are quite a few industries in which caustic soda is relevant. The application is based on its chemical and physical properties, which make this compound indispensable in many syntheses and processes.
There are several main areas in which sodium hydroxide is an essential element.
- Chemical production (synthesis of esters, soaps, fats, production of fibers, etching of aluminum, for the production of petroleum products, as a catalyst in many processes; is the main substance for neutralizing acids and their corresponding oxides; in analytical chemistry it is used for titration; also used to obtain pure metals, many salts, other bases and organic compounds).
- In the production of paper for processing wood pulp (getting rid of lignin from wood).
- Caustic soda is also indispensable in human economic activity. The use of numerous detergents and cleaning products based on it is very important. Soap making, shampoo production - all this cannot be done without caustic soda.
- Necessary for biofuel synthesis.
- It is used on a national scale for degassing and neutralizing toxic substances that affect organisms.
- Production of medicines and narcotic drugs.
- Food industry - confectionery, chocolate, cocoa, ice cream, coloring of sweets, olives, baking of bakery products.
- In cosmetology for the removal of foreign formations (moles, papillomas, warts).
- Used in distilleries and tobacco factories.
- In the textile industry.
- Production of glass: colored, regular, optical and others.
It is obvious that sodium hydroxide is a very important and useful substance in human activity. It is not in vain that it is synthesized in the world annually in tons - 57 million or more.
Chemical indicators of caustic soda (Table 1)
| No. | The name of indicators | Brand TR | Brand RD | |
| Top grade | First grade | |||
| 1 | Appearance | White scaly mass. Light color allowed | Colorless or colored liquid, crystallized sediment is acceptable | |
| 2 | Mass fraction of sodium hydroxide, %, not less | 98,5 | 46,0 | 44,0 |
| 3 | Mass fraction of sodium carbonate, %, no more | 0,8 | 0,6 | 0,8 |
| 4 | Mass fraction of sodium chloride, %, no more | 0,05 | 3,0 | 3,8 |
| 5 | Mass fraction of iron in terms of Fe2O3, %, no more | 0,004 | 0,007 | 0,02 |
| 6 | Mass fraction of sodium chlorate, %, no more | 0,01 | 0,25 | 0,3 |
| 7 | Mass fraction of mercury, %, no more | 0,0005 | — | — |
Physical properties
White powdery substance, sometimes colorless. It can be in the form of a fine crystalline powder or in the form of flakes. Most often in the form of large crystals. The melting point is quite low - 65.1 ° C. Absorbs moisture very quickly and transforms into the hydrated form of NaOH 3.5H2O. In this case, the melting point is even lower, only 15.5°C. Almost unlimitedly soluble in alcohols and water. It feels both solid and liquid soapy.
Very dangerous in concentrated and diluted form. It can damage all the membranes of the eye, including the optic nerves. Contact with eyes may result in blindness. Therefore, working with this connection is extremely dangerous and requires protective equipment.
Security measures
Caustic soda in its pure form is a very aggressive alkali that can cause severe chemical burns if it comes into contact with the skin or eyes. Therefore, if you decide to use it at home, be sure to take safety precautions. Regular kitchen gloves will not work. You need to use special rubberized gloves and safety glasses.
If caustic solution accidentally gets into your eyes, you should immediately rinse them under running water, and then consult a doctor. If it gets on the skin, then it needs to be wiped with a weak solution of vinegar to neutralize the effect of the alkali.
Store caustic soda in a tightly sealed container and in a hard-to-reach place so that children cannot get to it, and never forget to use protective equipment when using it at home.
Facebook
Storage conditions
Storage of caustic soda is carried out under certain conditions. This is because it is extremely reactive, especially when the room is damp. The main conditions are the following.
- Storage away from heating devices.
- Hermetically sealed and sealed packages that do not allow moisture to pass through.
- Dry crystalline caustic is stored in bags of a special composition (dense polyethylene), liquid - in dark glass containers with ground stoppers. If the quantity is large and requires transportation, then the caustic soda solution is placed in special steel containers and canisters.
This substance can be transported by any known method in compliance with safety regulations, excluding transportation by air.
Liquid sodium lye
In addition to crystalline, there is also an aqueous solution of caustic soda. Its formula is the same as for solid. Chemically, solutions are more applicable and convenient to use. Therefore, caustic is used more often in this form.
Caustic soda solution, the formula of which is NaOH, is used in all of the above areas. It is only inconvenient during transportation, since it is better to transport dry caustic. In all other properties it is in no way inferior to crystals, and in some even surpasses them.
How to treat a burn
Attention! If caustic soda gets into your eyes, rinse them with cool water for several minutes.
First aid for damaged skin includes several points:
- Washing off the alkali. The procedure must be carried out for at least 20 minutes. Only water can be used for cleaning. Wet wipes or a dampened towel are not suitable, as they may cause the reagent to penetrate deeper into the skin structure.
- Neutralization of alkali action. At this stage, the damaged area is treated with an aqueous solution of vinegar or lemon juice.
- Relieving pain. To implement this point, apply a bandage soaked in cold water to the burn site or take painkillers.
Attention! If the damaged skin becomes blistered, you should immediately call an ambulance.