We know from school that bases are compounds where metal atoms are bonded to one or more hydroxo groups - KOH, Ca(OH)2, etc. However, the concept of “base” is actually broader, and there are two theories of bases - protic (Brønsted-Lowry theory) and electronic (Lewis theory). We will consider Lewis bases and acids in a separate article, so we will take the definition from Brønsted’s theory (hereinafter in this article - only Brønsted bases): Bases (hydroxides) are substances or particles that can accept (split off) a proton from an acid. According to this definition, the properties of a base depend on the properties of the acid—for example, water or acetic acid behave like bases in the presence of stronger acids:
H2SO4 + H2O ⇄ HSO4— + H3O+ (hydronium cation)
H2SO4 + CH3COOH ⇄ HSO4— + CH3COOH2+
Classification of bases
The grounds can be classified according to the following criteria:
- Based on solubility, bases are divided into soluble alkalis (NaOH, KOH) and insoluble bases (Ca(OH)2, Al(OH)3).
- Based on acidity (the number of hydroxyl groups), bases are divided into monoacid (KOH, LiOH) and polyacid (Mg(OH2), Al(OH)3).
- According to their chemical properties, they are divided into basic (Ca(OH)2, NaOH) and amphoteric , that is, exhibiting both basic and acidic properties (Al(OH)3, Zn(OH)2).
- Based on strength (degree of dissociation) they are distinguished: a) strong (α = 100%) - all soluble bases NaOH, LiOH, Ba(OH)2, slightly soluble Ca(OH)2. b) weak (α < 100%) – all insoluble bases Cu(OH)2, Fe(OH)3 and soluble NH4OH.
Acids and alkalis in our body
To digest food, the body uses gastric juice, which contains hydrochloric acid and various enzymes. Sometimes, especially after overeating, we may feel pain in the stomach. Most often, to relieve discomfort, it is enough to take an antacid, or antacid, drug, the main effect of which is aimed at neutralizing hydrochloric acid in the stomach. As a rule, all antacids are alkalis, and they neutralize the increased activity of acids.
The effect of antacids
Share link
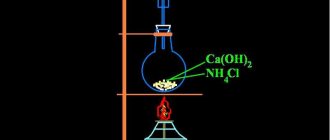
Receipt
Interaction of active metal with water:
2Na + 2H2O → 2NaOH + H2
Ca + 2H2O → Ca(OH)2 + H2
Mg + 2H2O Mg(OH)2 + H2
Interaction of basic oxides with water (only for alkali and alkaline earth metals):
Na2O + H2O → 2NaOH,
CaO + H2O → Ca(OH)2.
An industrial method for producing alkalis is the electrolysis of salt solutions:
2NaCI + 4H2O 2NaOH + 2H2 + CI2
The interaction of soluble salts with alkalis, and for insoluble bases this is the only way to obtain:
Na2SO4 + Ba(OH)2 → 2NaOH + BaSO4
MgSO4 + 2NaOH → Mg(OH)2 + Na2SO4.
Nomenclature
The names of the bases are constructed according to the rule: “hydroxide + name of the metal.” If the oxidation state of the metal is not constant, its name indicates its valence: iron(III) hydroxide.
Some grounds have, in addition to systematic ones, trivial (traditional and technical) names:
| Base | Trivial name |
| sodium hydroxide NaOH | sodium hydroxide; caustic (tech.) |
| potassium hydroxide KOH | caustic potassium; potassium lye |
| ammonia hydrate NH4OH (ammonium hydroxide in solution) | ammonia water |
| calcium hydroxide Ca(OH2) | slaked lime; fluffy |
| Barium hydroxide Ba (OH2) (in solution) | barite water |
Physical properties
All bases are solids that have different colors. Insoluble in water, except for alkalis.
Attention! Alkalis are very caustic substances. If they come into contact with the skin, alkali solutions cause severe, long-healing burns; if they come into contact with the eyes, they can cause blindness. When working with them, you should follow safety precautions and use personal protective equipment.
Appearance of the bases. From left to right: sodium hydroxide, calcium hydroxide, iron metahydroxide
Acids and alkalis in nature
You have already seen that there are a huge amount of acids and alkalis around us. Dairy products, vegetables and fruits contain citric, malic, oxalic, acetic, lactic, ascorbic and other acids. It’s hard to believe, but the seeds of cherries and almonds contain (albeit in minimal quantities) such a strong poison as hydrocyanic acid! It is known that many insects prefer to defend themselves with various acids. Ever wondered why the bites of the common tiny ant are so painful? And all because it injects droplets of formic acid into the wound. The same acid is also secreted by some types of caterpillars, and tropical spiders and some beetles protect themselves from enemies with the help of acetic and sulfuric acids.
CAREFULLY! As a rule, concentrated acids and alkalis are available in all school chemistry classrooms, and they can only be used under the guidance of a teacher.
Chemical properties
The chemical properties of bases from the point of view of the theory of electrolytic dissociation are determined by the presence in their solutions of an excess of free hydroxide - OH- ions.
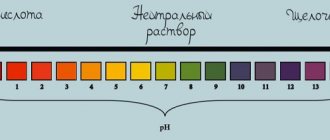
Changing indicator colors:
phenolphthalein – raspberry
litmus - blue
methyl orange – yellow
Phenolphthalein gives the alkali solution a crimson color
Reaction with acids to form salt and water (neutralization reaction):
2KOH + H2SO4 → K2SO4 + 2H2O,
soluble
Mg(OH)2 + 2HCI → MgCI2 + 2H2O.
insoluble
Interaction with acid oxides:
2KOH + SO3 → K2SO4 + H2O
Interaction with amphoteric oxides and hydroxides:
a) when melting:
2NaOH + AI2O3 → 2NaAIO2 + H2O,
NaOH + AI(OH)3 → NaAIO2 + 2H2O.
b) in solution:
2NaOH + AI2O3 +3H2O → 2Na[AI(OH)4],
NaOH + AI(OH)3 → Na[AI(OH)4].
Interaction with some simple substances (amphoteric metals, silicon and others):
2NaOH + Zn + 2H2O → Na2[Zn(OH)4] + H2
2NaOH + Si + H2O → Na 2SiO3 + 2H2
Interaction with soluble salts with the formation of precipitation:
2NaOH + CuSO4 → Cu(OH)2 + Na2SO4,
Ba(OH)2 + K2SO4 → BaSO4 + 2KOH.
Acids in soil
It turns out that there are acids in soils, and the ability of soil to exhibit the properties of acids is called acidity. This indicator depends on the presence of hydrogen ions in the soil. The growth and development of plants depends on the acidity of the soil. Most of them prefer neutral or close to neutral soils. However, there are a number of plants that thrive in acidic soils, such as rhododendrons, hydrangeas, and azaleas. Some hydrangea varieties may change bud color depending on growing conditions and soil acidity. Scientists have found that the color of buds is affected by the presence of aluminum!
Most garden soils are characterized by sufficient content of this element. In an acidic environment, aluminum compounds become soluble and become available to plants, which is why blue buds grow. In a neutral or alkaline environment, aluminum is in the form of insoluble compounds, which is why it does not enter plants. As a result, pink buds grow on such soils.
Ethylamine
Formula: C2H5NH2 Basicity (pKb): 3.35
Ethylamine is a corrosive primary amine. It is a weak base, meaning that it does not ionize completely in aqueous solution to form ethyl ammonium cations and hydroxide anions.
Equilibrium is established between non-ionized ethylamine molecules and two ions that are formed as a result of its ionization.
Like other primary amines, ethylamine is an excellent solvent for lithium metal. These solutions are used to reduce unsaturated organic substances such as alkynes and naphthalenes.
In addition, ethylamine produces toxic nitrogen oxides when burned. It is usually stored in a closed container; however, prolonged exposure to high temperatures can cause it to rupture abruptly.
Pyridine
Formula: C5H5N Basicity (pKb): 8.75
The structure of pyridine is very similar to that of benzene, but one methine group is replaced by a nitrogen atom. The presence of nitrogen (and its lone pair) in the benzene ring makes pyridine a unique compound in chemistry.
Pyridine is a significantly weaker base than alkylamines and typical aliphatic tertiary amines. It is a water-soluble and flammable liquid with an unpleasant “fish-like” odor. Although pure pyridine is colorless, impure or old samples may appear yellow.
This base is primarily used to dissolve other compounds and make a variety of products, including dyes, paints, insecticides, drugs, food flavorings, vitamins, adhesives, and rubber products. It is also found in many natural materials in the environment.
Trimethylamine
Formula: N(CH3)3 Basicity (pK b): 4.20
Trimethylamine is a colorless tertiary amine in which each hydrogen atom is replaced by a methyl group. The central nitrogen atom is attached to three methyl groups in a trigonal pyramidal geometry.
Trimethylamine is a gas at room temperature, but is highly soluble in water. It is usually sold as a 40% solution in water. At lower concentrations it smells like rotting fish. At higher concentrations it smells like ammonia. Short-term inhalation of high concentrations or long-term inhalation of low concentrations can cause serious health problems.
The compound is an excellent nucleophile, meaning that it can form bonds with electrophiles by donating an electron pair. It has several industrial uses - it is mainly used for the synthesis of plant growth regulators, dye leveling agents, tetramethylammonium hydroxide and choline.
Methylamine
Formula: CH3NH2 Basicity (pKb): 3.34
Methylamine is a common aliphatic primary amine in which NH2 is attached to the carbon chain. All aliphatic primary amines, including this one, are stronger bases than ammonia.
As can be seen from the formula, the main difference between ammonia and methylamine is the presence of the CH3 group in the latter. Because the alkyl group pushes electrons away from itself, the nitrogen atom accumulates a small negative charge, making its lone pair even more attractive to hydrogen ions.
And because ammonia does not have an electron-donating group, it is a weaker base than methylamine (in which the nitrogen has a more negative charge, so it accepts H+ more readily).
Like ammonia, methylamine is a colorless gas or liquid with a pungent odor. It can easily catch fire. If exposed to high temperatures for a long time, methylamine containers may rupture. Therefore, it must be handled with care.
Methylamine is sold primarily as a solution in tetrahydrofuran, ethanol, methanol, or as an anhydrous gas in pressurized containers. It is widely used to produce pesticides, surfactants, pharmaceuticals, paint thinners and rubber chemicals.
Aniline
Formula: C6H5NH2 Basicity (pKb): 9.13
Aniline is the simplest aromatic amine, which is a yellow-brownish oily liquid with a musty fishy odor. It contains an amine attached to a benzene ring.
More specifically, the lone pair above the nitrogen atom in the NH2 group is conjugated with the Pi electron of the benzene ring. Therefore, aniline cannot easily lose an electron pair, making it a weak base.
It is highly soluble in alcohol and ether and slightly soluble in water. When reacting with strong acids, it forms anilinium ions.
Aniline is toxic in nature. It is quickly absorbed into the skin, lungs and gastrointestinal tract of experimental animals. However, it is widely used for the synthesis of chemicals, especially agricultural chemicals, photochemicals and dyes.
Propylamine
Formula: C3H7NH2 Basicity (pKb): 3.45
Propylamine belongs to a class of organic compounds called monoalkylamines. These compounds contain a primary aliphatic amine group.
Propylamine is soluble in water and has a lower density than water. It is a colorless volatile liquid, and its vapors are heavier than air. When burned, it produces toxic nitrogen oxides.
Propylamine is typically found in lower concentrations in several different foods, such as green bell peppers, orange bell peppers, and in highest concentrations in red bell peppers and yellow bell peppers. Researchers have also found it in wild celery and common grapes.
In the laboratory, propylamine hydrochloride is prepared by mixing ammonium chloride with 1-propanol at high pressure and temperature using a Lewis acid catalyst such as ferric chloride.
Propylamine is primarily used for the synthesis and analysis of other chemicals.
Hydrazine
Formula: N2H4 Basicity (pK b): 8.1
Hydrazine is a compound containing two nitrogen atoms with a single bond and four peripheral hydrogen atoms. Its aqueous solution (concentration greater than 37%) is colorless, corrosive and toxic if ingested and absorbed through the skin.
When hydrazine is mixed with water, it forms a denser monohydrate (1.032 g/cm3) than the anhydrous substance.
N 2 H 4 + H 2 O -> + + OH —
Like ammonia, hydrazine has basic (alkaline) chemical properties. It is a highly reactive base and reducing agent used in a wide range of medical and industrial applications.
About 100.00 metric tons of hydrazine are produced annually worldwide. It is mainly used as a blowing agent for the production of polystyrene foam.
It is also used as a rocket fuel - when the fuel burns, hydrazine decomposes into gaseous ammonia, nitrogen and hydrogen, releasing large amounts of thermal energy. Hydrogen and nitrogen gases are forced out of the rocket through a nozzle to create thrust.