Baking soda is an alkaline substance that is familiar to almost everyone. There are several types of sodium compound, the most common of which are baking soda, soda ash and caustic soda. If baking soda is in most cases used for cooking and medical purposes, then caustic and soda ash are more often used in everyday life, as cleaning agents and detergents. These active substances are also widely used in industry, as the main components of the production of consumer goods.
Differences in the properties of soda are determined by differences in the structure of the molecules, which are reflected in the chemical formulas
Types of substances
All types of product are sodium. But their properties are different, which determines the methods of their use.
There are several types of this substance:
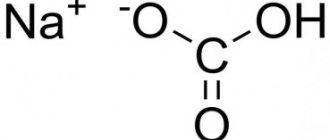
- Food. Used for baking. It is a fine-crystalline powder, white, odorless. Formula - NAHCO3. It is also called bicarbonate, bicarbonate, sodium bicarbonate.
- Calcined (Na2CO3). The material is white with a slightly grayish tint, finely crystalline. It has been given several names: sodium carbonate, linen, sodium salt of carbonic acid. For production, dehydration (calcination) technology is used.
- Caustic or sodium hydroxide (NaOH). Also called caustic soda, for its ability to quickly corrode the skin. It is white in color, has a solid crystal structure, and is odorless. It has increased hygroscopicity and is able to absorb moisture from the air. When dissolved, it undergoes a violent reaction with the release of heat, the water becomes soapy.
Safety regulations
When working with alkali, you need to follow a number of precautions. It is necessary to ensure air flow into the room for quality ventilation.
It is necessary to use gloves, glasses and clothing that will protect the skin and eyes from contact with the caustic substance. It is advisable to use a cotton-gauze bandage or a respirator. It is imperative to ensure that the caustic does not come into contact with metals, because this may cause unwanted reactions.
When using soda, you must follow the recommended proportions for preparing solutions. This is especially important if you plan to use a dishwashing and laundry detergent.
Caustic soda can only be stored in containers that do not react with this substance. It is best to use the original packaging. It should be borne in mind that soda is volatile, so the container in which it will be stored must be tightly closed. The product should be stored in a place inaccessible to children and animals.
Application and difference of forms
Soda ash and caustic soda have found their application in solving the household needs of the population. They are used as cleaning and detergents.
In addition, they are freely used in various industrial sectors, in the production of goods of various consumption.
Sodium carbonic acid is made from caustic! This is one of the ways (it is also obtained from natural raw materials, nephelines, sodium chloride).
Carbonation is the result of various chemical processes occurring under the influence of high temperatures. From what sodium hydroxide turns into sodium carbonate.
After undergoing heat treatment, caustic soda and its derivative, calcined, acquire distinctive characteristics.
Recommended for you:
What is bread soda, benefits and harm, how to use
The main differences lie in different properties, areas of application, and crystal structure.
Sodium carbonate is available in the form of a homogeneous powder or small granules. It is the basis for the manufacture of cleaning products, washing dishes, various surfaces, and containers. Detergents based on Na2CO3 easily dissolve fats, oils, and lime deposits.
Expert opinion
Advice!
Soda ash is an excellent bioregulator for alkalizing an acidic environment. With its help, you can soften water and prevent the formation of lime deposits in washing machines and water heating tanks.
In addition, sodium carbonate is used in glass production. It is an essential component during the glass production operation.
Also indispensable in the manufacture of paper, cardboard, other cellulose products, and the production of petroleum products
Other areas
The scope of application of sodium hydroxide, due to its unique ability to corrode any dirt and leave behind sterile cleanliness, is quite extensive: at home, in your summer cottage, and even in livestock farming.
- Rust. To return the pipes to their original appearance, they are first treated with acidic agents, and then a mixture of caustic soda, formalin and ammonium diluted with water (in equal proportions) is applied.
- Stains from fuel oil and ingrained fat. To rid things of particularly heavy stains, soak and leave them for two to three hours in a solution of 2% caustic soda (a tablespoon per liter of water). After that, wash as usual. Particularly daring housewives even use caustic soda in an automatic machine. Three to five tablespoons are poured directly into the drum and a cycle is started with a temperature of 50-100oC. If you are going to wash by hand, then the proportions are three tablespoons of caustic solution per 10 liters of water. You should not try to clean wool and silk fabrics in this way - irreparable damage will be caused to delicate materials.
- Washing floors. For 10 liters of liquid you need three tablespoons of a powdery substance. Wipe the surface with the solution, then run over it with clean water and wipe everything dry.
- Disinfection. If livestock becomes ill, the premises where the animals are kept are treated with a 4% caustic solution.
- Plant processing. Gardeners use caustic soda (five tablespoons per bucket of water) to treat the bushes. This process will not give caterpillars, gray mold and powdery mildew a chance to survive. Caustic acid can accumulate inside the soil. Therefore, such procedures should not be carried out regularly.
Obtaining the substance
There are about sixty natural deposits on the planet for the extraction of material for the production of Na2CO3.
These are, as a rule, soda lakes, located mostly on the American continent. They are also found in the territories beyond Lake Baikal and the western regions of Siberia.
In addition, the ashes of burnt algae and plants growing on the shores of reservoirs are used to obtain the product.
Today, four methods are used to obtain Na2CO3:
- Ammoniacal (from sodium chloride). The method is costly and environmentally unsafe.
- Using natural soda raw materials. Salt water and sediments (brine) from estuaries, lakes, and artificial reservoirs are processed.
- Using ore containing nepheline. When processing raw materials, Na2CO3 and potash are obtained.
- Carbonation of sodium hydroxide. That is, the production of soda ash from caustic soda.
Of all the above methods, the cheapest and most environmentally friendly is the use of natural soda raw materials.
Where not to use
There are materials for which contact with soda ash is strictly contraindicated. For example, paint may come off after such treatment. Also, do not wash suspended ceilings with alkali. Four more surfaces that don’t “like” laundry soda.
- Delicate materials. Sodium carbonate is harmful to silk, wool, leather, impregnated fabrics, membrane surfaces, leatherette and suede.
- Aluminum, cast iron and plastic. Aluminum and cast iron have already been discussed above. Also, do not use the product for general cleaning of the refrigerator. When exposed to strong alkali, plastic can deteriorate.
- Wood, varnish. Sodium carbonate is harmful to the surfaces of wooden and lacquered furniture: the polish may be damaged.
- Fiberglass. Bricks and tiles made from fiberglass also do not like soda ash.
Using soda ash
Na2CO3 is successfully used for a variety of household purposes. Sodium carbonate, when added to water, will reduce hardness.
Washing powders and other detergents are made with the addition of this substance. Housewives successfully use it when washing dishes.
But you must take precautions and protect the skin of your hands with rubber gloves.
- The dishwashing solution is prepared as follows: take 3 tbsp. l. soda ash, dissolve in 3 liters of hot water.
Recommended for you:
Baking soda: beneficial properties, use and treatment
This solution is enough to wash a significant amount of dirty dishes. Afterwards, clean plates must be rinsed in warm water.
There is no point in using additional detergents, since the Na2CO3 solution solves the problem 100%. It not only degreases, but also helps get rid of negative microorganisms.
A real scourge for household appliances intended for washing and heating water is the presence of lime deposits.
To prevent scale, use a mixture of sodium carbonic acid at least twice a month.
- You need to do this as follows: pour 5 tbsp into the laundry chamber or inside the water heating container. l. baking soda.
- Switch the washing machine to washing mode, heat the tank to 90 °C, and let the unit run for a while. After completing the process, rinse the equipment with a strong stream of water.
Na2CO3 can be used for washing dirty clothes, oily overalls or clothes.
- The solution is prepared as follows: add 1 to 3 tbsp to the receiving chamber of the washing machine, and in the case of hand washing, add 1 to 3 tbsp to a bowl of water. l. soda
The dose should be adjusted based on the degree of soiling of the clothing, the persistence of the stains, and their number. In this situation, it is necessary to strengthen the washing powder with sodium carbonate.
But it must be used in doses, as it makes the water soft, which increases the volume of soap suds.
The listed methods of application are the most common and most used. Although the potential of the substance is very great, it can have a wider range of circulation.
How is it different from food
What is the difference between soda ash and baking soda? At least because food grade can be used in cooking, but sodium carbonate cannot. There are four more differences.
- Alkaline reaction. In sodium carbonate it is more powerful - ph11, in baking soda - ph8.
- View. Sodium carbonate has a different composition and a looser structure.
- Power of influence. The non-food compound is usually used to clean areas that are too dirty and that regular baking soda can't handle.
- Safety . Although soda ash is not as dangerous as caustic soda, you still need to take precautions when interacting to protect your skin, nose, mouth and eyes.
Application of caustic soda
This substance is produced in two versions: in the form of small white flakes, very hygroscopic, quickly dissolving, and in the form of a colorless concentrated liquid.
NaOH is used in:
- industrial production for neutralizing various acids;
- pharmaceuticals in the manufacture of the disinfectant chloramine;
- home conditions for cleaning sewer systems.
Recommended for you:
Is baking soda an antiseptic?
To clean the drains, you need 5 or 7 tbsp. l. Pour caustic soda into the sewer pipe and pour hot water on top.
For about 2-3 hours, it is advisable to refrain from using the sewer and do not drain the water. A similar sequence can be carried out both when cleaning contaminated pipes and as preventive measures.
Cleaning the cesspool
Peculiarities. Getting caustic into the soil has a negative effect on the soil. Therefore, the pit must be absolutely sealed and have a proper ventilation system.
Your actions
- Prepare the solution according to the same principle as described above for sewer pipes. Depending on the size of the pit, 2-4 kg of caustic soda will be required.
- Fill the drainage hole with the mixture.
- As a rule, the active period of the product lasts about four minutes.
- The cycle must be repeated twice.
Reviews
Stepan Vladimirovich, 54 years old
Sodium carbonate is simply irreplaceable in the household. For example, my wife always washes my work clothes (I work in a carriage building shop) with this substance. It removes stains from industrial oils with a bang. Recently I had to clean such dirt on a car seat. It turned out great.
Maxim Sergeevich, 36 years old
It was necessary to clean the old radiator from the stove. They suggested caustic soda for this purpose. Cleaned it well, removed dirt and oil quickly. In principle, that’s what was required.
Tamara Sergeevna, 48 years old
I have been using soda ash almost all my life. My mother also used it. A very good product for cleaning tiles, dishes, stoves, and sinks. Better than all these newfangled products.
Precautionary measures
Soda ash is not nearly as safe as baking soda. Therefore, always work with gloves, and after each procedure, do not forget to thoroughly rinse off any remaining product with water. Also follow the following three rules.
- Further from products. Do not store laundry soda with food, and keep it away from children and four-legged pets.
- Eyes and skin. If lye splashes into your eye or gets on your skin, rinse the area with plenty of clean water.
- Use a respirator. Too much sodium carbonate can cause respiratory irritation. If there is no protective mask, perform all manipulations with an outstretched arm.
Now you know what soda ash is needed for. However, the scope of its application is very wide and is not limited to everyday life. Its properties are used in livestock farming and industrial production. Judging by the reviews, robes and overalls washed with sodium carbonate look like new.